Exploring Octapharma Careers: Impact Patients’ Lives Through Your Work
Introduction
Octapharma careers provide a unique opportunity to combine your passion for healthcare with the flexibility and growth potential of remote work. As a leading global pharmaceutical company specializing in human proteins, Octapharma is dedicated to improving the lives of patients with rare and severe diseases. In this article, we’ll explore the diverse range of remote job opportunities available at Octapharma, catering to various skills and backgrounds. Whether you’re a seasoned professional in the pharmaceutical industry or looking to make a meaningful impact in a new field, Octapharma has a career path that could be perfect for you.

Octapharma Remote Job Opportunities
- Remote Clinical Research Associate
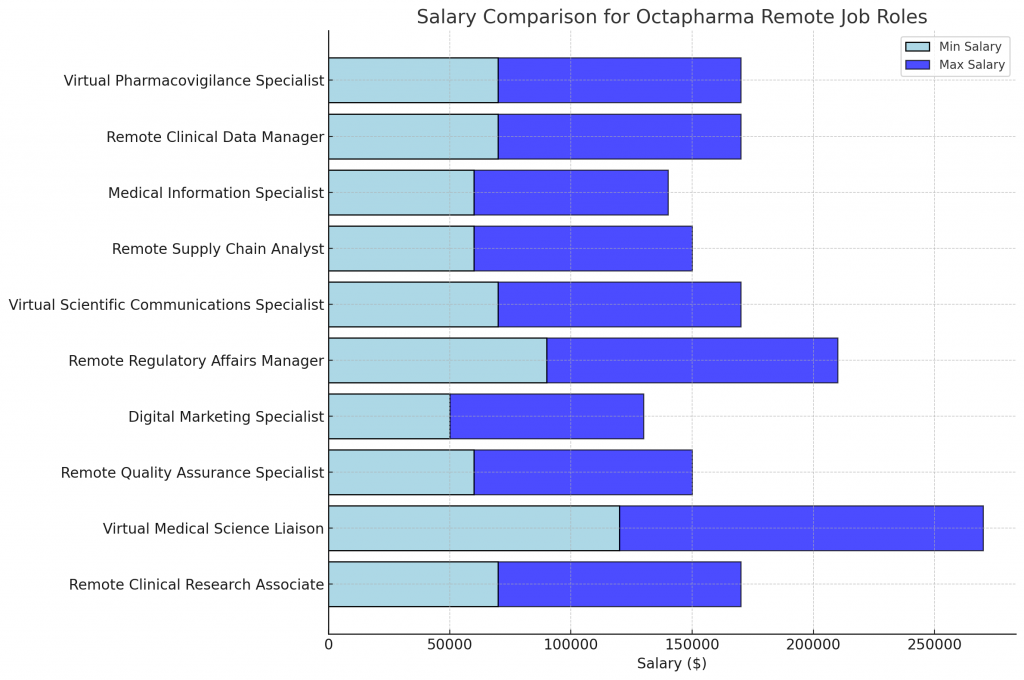
- Job Description: Coordinate and monitor clinical trials, ensuring compliance with protocols and regulations. Collaborate with investigators, review data, and maintain study documentation.
- Requirements: Bachelor’s degree in a scientific field, experience in clinical research, and strong organizational and communication skills. Knowledge of GCP and FDA regulations is a plus.
- Estimated Salary: $70,000-$100,000/year
- Benefits: Opportunity to work on groundbreaking research, contribute to patient care, and enjoy a flexible remote schedule
- Virtual Medical Science Liaison
- Job Description: Serve as a scientific expert, providing medical information and support to healthcare professionals. Develop and maintain relationships with key opinion leaders and stay current on scientific advancements.
- Requirements: Advanced degree in a scientific field (Ph.D., PharmD, or MD), excellent communication and presentation skills, and the ability to translate complex scientific information. Industry experience is a plus.
- Estimated Salary: $120,000-$150,000/year
- Benefits: Opportunity to be at the forefront of medical science, impact patient care, and work with a diverse range of healthcare professionals
- Remote Quality Assurance Specialist
- Job Description: Ensure the quality and compliance of Octapharma’s products and processes. Conduct audits, review documentation, and collaborate with cross-functional teams to maintain high standards.
- Requirements: Bachelor’s degree in a scientific or technical field, experience in quality assurance, and knowledge of cGMP and FDA regulations. Attention to detail and problem-solving skills are essential.
- Estimated Salary: $60,000-$90,000/year
- Benefits: Opportunity to ensure the safety and efficacy of life-saving therapies, work with a dedicated team, and grow your career in a remote setting
- Digital Marketing Specialist
- Job Description: Develop and execute digital marketing strategies to promote Octapharma’s products and services. Manage social media, create content, and analyze campaign performance.
- Requirements: Bachelor’s degree in marketing or a related field, experience in digital marketing, and strong creative and analytical skills. Knowledge of the healthcare industry is a plus.
- Estimated Salary: $50,000-$80,000/year
- Benefits: Opportunity to shape brand awareness, work with a talented marketing team, and impact patient lives through compelling storytelling
- Remote Regulatory Affairs Manager
- Job Description: Ensure compliance with regulatory requirements for Octapharma’s products. Prepare and submit regulatory filings, interact with health authorities, and provide strategic guidance to cross-functional teams.
- Requirements: Bachelor’s degree in a scientific or regulatory field, experience in regulatory affairs, and knowledge of global regulatory requirements. Strong project management and communication skills are essential.
- Estimated Salary: $90,000-$120,000/year
- Benefits: Opportunity to navigate complex regulatory landscapes, contribute to patient access to therapies, and advance your career in a remote setting
- Virtual Scientific Communications Specialist
- Job Description: Develop and disseminate scientific content, including publications, presentations, and educational materials. Collaborate with medical affairs and cross-functional teams to ensure scientific accuracy and consistency.
- Requirements: Advanced degree in a scientific field (Ph.D. or MD), excellent writing and editing skills, and the ability to translate complex scientific information for various audiences. Knowledge of the pharmaceutical industry is a plus.
- Estimated Salary: $70,000-$100,000/year
- Benefits: Opportunity to communicate groundbreaking science, impact healthcare professional education, and grow your career in a remote setting
- Remote Supply Chain Analyst
- Job Description: Analyze and optimize Octapharma’s supply chain processes to ensure the efficient and timely delivery of products. Monitor inventory levels, forecast demand, and collaborate with cross-functional teams.
- Requirements: Bachelor’s degree in supply chain management, business, or a related field, experience in supply chain analysis, and strong analytical and problem-solving skills. Knowledge of the pharmaceutical industry is a plus.
- Estimated Salary: $60,000-$90,000/year
- Benefits: Opportunity to drive operational excellence, work with a global team, and impact patient access to life-saving therapies
- Medical Information Specialist
- Job Description: Provide accurate and timely medical information to healthcare professionals and patients. Respond to inquiries, review literature, and maintain medical information databases.
- Requirements: Bachelor’s degree in a scientific or medical field, experience in medical information or a related role, and excellent communication and research skills. Knowledge of pharmacovigilance is a plus.
- Estimated Salary: $60,000-$80,000/year
- Benefits: Opportunity to be a trusted resource for medical information, work with a dedicated team, and impact patient care in a remote setting
- Remote Clinical Data Manager
- Job Description: Manage clinical trial data, ensuring accuracy, completeness, and compliance with protocols and regulations. Develop and maintain data management plans, oversee data entry and validation, and collaborate with cross-functional teams.
- Requirements: Bachelor’s degree in a scientific or technical field, experience in clinical data management, and knowledge of clinical trial processes and regulations. Strong attention to detail and problem-solving skills are essential.
- Estimated Salary: $70,000-$100,000/year
- Benefits: Opportunity to ensure data integrity, contribute to clinical research, and advance your career in a remote setting
- Virtual Pharmacovigilance Specialist
- Job Description: Monitor and assess the safety of Octapharma’s products. Review adverse event reports, conduct safety analyses, and collaborate with cross-functional teams to ensure patient safety.
- Requirements: Bachelor’s degree in a scientific or medical field, experience in pharmacovigilance, and knowledge of safety regulations and reporting requirements. Strong analytical and communication skills are essential.
- Estimated Salary: $70,000-$100,000/year
- Benefits: Opportunity to ensure patient safety, work with a dedicated team, and impact the safe use of life-saving therapies
To apply for any of these jobs or similar positions, click here.
What People Say
“Working remotely as a Clinical Research Associate at Octapharma has been an incredible experience. I have the opportunity to work on groundbreaking research that directly impacts patients’ lives. The company provides a supportive and collaborative environment, even in a remote setting. The salary and benefits are competitive, and the work-life balance is excellent. The only challenge is the occasional travel to clinical sites, but the opportunity to make a difference makes it worthwhile.”
John Hawthorne, Mystic, Connecticut
“Being a Virtual Medical Science Liaison at Octapharma has allowed me to combine my scientific expertise with my passion for educating healthcare professionals. I enjoy staying at the forefront of medical advancements and translating complex information into actionable insights. The remote work arrangement has been seamless, and the company provides all the necessary resources for success. The salary and benefits package is attractive, and the opportunity to impact patient care is truly rewarding.”
Kimberly Ashford, Whitefish, Montana
“As a Remote Quality Assurance Specialist at Octapharma, I play a crucial role in ensuring the quality and safety of our products. The company’s commitment to maintaining the highest standards is inspiring, and I’m proud to be part of a team that puts patients first. The remote work setup allows for a great work-life balance, and the company provides ongoing training and support. The only challenge is the occasional long hours during audits or inspections, but the satisfaction of knowing I’m contributing to the quality of life-saving therapies makes it all worthwhile.”
Ethan Smith, Sandpoint, Idaho
“Working as a Remote Regulatory Affairs Manager at Octapharma has been a challenging and rewarding experience. I enjoy navigating the complex regulatory landscape and ensuring our products meet all the necessary requirements. The company provides a supportive and collaborative environment, even in a remote setting. The salary and benefits are competitive, and the opportunity to impact patient access to therapies is gratifying. The main challenge is staying up-to-date with ever-changing regulations, but Octapharma provides the resources and training to help us stay ahead of the curve.”
Alexander Davis, Ludington, Michigan
“Being a Virtual Pharmacovigilance Specialist at Octapharma has allowed me to make a real impact on patient safety. I take pride in monitoring and assessing the safety of our products, ensuring that patients receive the highest quality care. The remote work arrangement has been a great fit for my lifestyle, and the company provides all the necessary tools and support for success. The salary and benefits are fair, and the work itself is meaningful. The only drawback is the occasional need to work outside regular hours to address urgent safety issues, but the opportunity to safeguard patient well-being makes it worthwhile.”
Lisa Aldridge, Wanaka, New Zealand
More Remote Jobs
- Remote Biostatistician
- Job Description: Design and analyze clinical trial data, provide statistical expertise, and collaborate with cross-functional teams to support drug development and regulatory submissions.
- Requirements: Advanced degree in statistics, biostatistics, or a related field, experience in clinical trial analysis, and proficiency in statistical programming languages (e.g., SAS, R). Knowledge of the pharmaceutical industry is a plus.
- Estimated Salary: $80,000-$120,000/year
- Benefits: Opportunity to apply statistical expertise, impact clinical research, and grow your career in a remote setting
- Virtual Medical Writer
- Job Description: Write and edit scientific and medical content, including regulatory documents, clinical study reports, and manuscripts. Collaborate with cross-functional teams to ensure accuracy and consistency.
- Requirements: Advanced degree in a scientific or medical field (Ph.D. or MD), excellent writing and editing skills, and the ability to interpret and communicate complex scientific information. Knowledge of the pharmaceutical industry is a plus.
- Estimated Salary: $70,000-$100,000/year
- Benefits: Opportunity to communicate scientific breakthroughs, impact medical knowledge, and advance your career in a remote setting
- Remote GxP Auditor
- Job Description: Conduct internal and external audits to ensure compliance with GxP regulations (e.g., GMP, GCP, GLP). Identify areas for improvement, provide recommendations, and collaborate with cross-functional teams to maintain quality standards.
- Requirements: Bachelor’s degree in a scientific or technical field, experience in GxP auditing, and knowledge of relevant regulations and guidelines. Strong communication and problem-solving skills are essential.
- Estimated Salary: $80,000-$120,000/year
- Benefits: Opportunity to ensure regulatory compliance, drive continuous improvement, and grow your career in a remote setting
- Clinical Trial Coordinator
- Job Description: Coordinate and support clinical trial activities, including patient recruitment, data collection, and site management. Ensure compliance with protocols and regulations, and collaborate with cross-functional teams.
- Requirements: Bachelor’s degree in a scientific or medical field, experience in clinical trial coordination, and strong organizational and communication skills. Knowledge of GCP and FDA regulations is a plus.
- Estimated Salary: $50,000-$80,000/year
- Benefits: Opportunity to support groundbreaking research, impact patient lives, and advance your career in a remote setting
- Remote Health Economics and Outcomes Research (HEOR) Specialist
- Job Description: Conduct economic evaluations and outcomes research to support the value proposition of Octapharma’s products. Analyze real-world data, develop economic models, and communicate findings to stakeholders.
- Requirements: Advanced degree in health economics, outcomes research, or a related field (Ph.D. or MS), experience in HEOR, and strong analytical and communication skills. Knowledge of the pharmaceutical industry is a plus.
- Estimated Salary: $80,000-$120,000/year
- Benefits: Opportunity to demonstrate the value of life-saving therapies, impact market access, and grow your career in a remote setting
- Virtual Scientific Communications Manager
- Job Description: Develop and implement scientific communication strategies, including publication planning, congress activities, and medical education. Collaborate with cross-functional teams to ensure scientific accuracy and consistency.
- Requirements: Advanced degree in a scientific field (Ph.D. or MD), experience in scientific communications, and excellent project management and communication skills. Knowledge of the pharmaceutical industry is a plus.
- Estimated Salary: $90,000-$130,000/year
- Benefits: Opportunity to shape scientific discourse, impact healthcare professional education, and advance your career in a remote setting
- Remote Regulatory Intelligence Analyst
- Job Description: Monitor and analyze regulatory developments and trends, assess their impact on Octapharma’s products and strategies, and provide insights to cross-functional teams.
- Requirements: Bachelor’s degree in a scientific, regulatory, or related field, experience in regulatory intelligence, and strong analytical and communication skills. Knowledge of global regulatory environments is a plus.
- Estimated Salary: $70,000-$100,000/year
- Benefits: Opportunity to stay at the forefront of regulatory developments, impact strategic decision-making, and grow your career in a remote setting
- Medical Affairs Manager
- Job Description: Develop and implement medical affairs strategies, engage with healthcare professionals and key opinion leaders, and provide scientific support for Octapharma’s products.
- Requirements: Advanced degree in a scientific or medical field (Ph.D., PharmD, or MD), experience in medical affairs, and excellent communication and relationship-building skills. Knowledge of the pharmaceutical industry is a plus.
- Estimated Salary: $120,000-$160,000/year
- Benefits: Opportunity to shape medical strategies, impact patient care, and advance your career in a remote setting
- Remote Clinical Data Scientist
- Job Description: Analyze clinical trial data, develop predictive models, and generate insights to support drug development and clinical decision-making. Collaborate with cross-functional teams to optimize trial design and outcomes.
- Requirements: Advanced degree in data science, statistics, or a related field (Ph.D. or MS), experience in clinical data analysis, and proficiency in programming languages (e.g., Python, R). Knowledge of the pharmaceutical industry is a plus.
- Estimated Salary: $100,000-$140,000/year
- Benefits: Opportunity to leverage data to drive innovation, impact clinical research, and grow your career in a remote setting
- Virtual Patient Advocacy Manager
- Job Description: Develop and maintain relationships with patient advocacy groups, gather patient insights, and ensure the patient perspective is integrated into Octapharma’s strategies and initiatives.
- Requirements: Bachelor’s degree in a relevant field, experience in patient advocacy or a related role, and excellent communication and relationship-building skills. Knowledge of rare diseases and the pharmaceutical industry is a plus.
- Estimated Salary: $80,000-$120,000/year
- Benefits: Opportunity to be a voice for patients, impact company strategies, and make a meaningful difference in patients’ lives
To apply for any of these jobs or similar positions, click here.

Conclusion
Octapharma careers offer a unique opportunity to make a difference in patients’ lives while enjoying the benefits of remote work and professional growth. From clinical research and regulatory affairs to medical communications and patient advocacy, there is a wide range of positions available for professionals with diverse skills and backgrounds.
To stay informed about the latest Octapharma job openings and receive expert advice on succeeding in the pharmaceutical industry, we encourage you to join our email list. As a subscriber, you’ll be the first to know about new remote job listings, get insider tips on crafting a standout application, and learn strategies for thriving in your Octapharma career.
Don’t miss out on the opportunity to join a global leader in the plasma-derived protein therapeutics industry and make a meaningful impact on patients’ lives. Subscribe to our email list today and take the first step towards your dream Octapharma career. Your future in the pharmaceutical industry awaits!

